Current Research Projects
POST-TBI OPIOID EXPOSURE EXACERBATES CHRONIC INJURY-INDUCED BEHAVIORAL AND NEUROINFLAMMATORY OUTCOMES
An estimated 330,000 service personnel have been diagnosed with traumatic brain injury (TBI) since the start of Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF). Of those sustaining TBIs, 70-80% are also treated for pain, most commonly with opioids. While overall prevalence of prescription opioid use among OEF/OIF/Operation New Dawn Veterans is relatively modest, reports suggest that initiation and chronic use of opioids increases proportionally with TBI severity, and that this may be linked to increased opioid misuse. The disproportionate use of opioids in Veterans with TBI remained after adjustment for sociodemographic characteristics, military service, pain disability and prior non-opioid therapy, making it inconsistent with clinical guidelines that caution against prescribing opioids for chronic pain in individuals with a history of TBI. Complications related to pain and reward have a negative impact on functional recovery following TBI. Sustaining a moderate-to-severe TBI increases prevalence of psychiatric disorders, which commonly emerge within the first-year post-injury and substance abuse is the 3rd most common diagnosis post-TBI, behind generalized anxiety and mood disorders. Chronic pain is the most frequently reported complaint after injury in Veterans and is directly associated with poorer quality of life and functional recovery.
EFFECTS OF LONGTERM MORPHINE TREATMENT ON OPIOID RECEPTOR SIGNALING AND INFLAMMATION IN THE CHRONIC POST-TBI PERIOD
Approximately 70-80% of military service members treated for traumatic brain injury (TBI) also receive pain management. Despite clinical guidelines cautioning against the use of opioid-based therapies in this patient population, studies conducted in the Veteran's Administration (VA) indicate that patients with moderate to severe TBI actually recieve opioids at a higher rate than non-TBI patients. Morphine is a commonly used opioid analgesic in the clinic and thus will be the opioid of focus in the current study. The proposed project will use an animal model of TBI to identify how combined exposure to brain injury and morphine affect pain behavior and two targets of opioid action in the brain, opioid receptor signaling and neuroinflammation (immune system activation). Due to the complexity of the neuronal and behavioral processes assessed, these studies rely on intact living systems. Further, this proposal will establish if pain behavior can be reduced by inhibition of inflammation with ibudilast (currently in clinical trials for multiple sclerosis). Together, this project addresses the critical need for an understanding of the mechanisms by which opioids administered post-TBI might alter later pain states and uphold the mission for VA patient care and rehabilitation to improve/expand the effective treatment options for individuals suffering from TBI.
HISTONE DEACETYLASE REGULATION OF COGNITIVE OUTCOMES AFTER MILD TBI
Mild traumatic brain injury (mTBI) affects millions of Americans every year, but active duty and reserve service members are at an increased risk of sustaining a TBI compared to civilians due to combat-related activities. Although symptoms of mTBI are usually short-lived, mTBI can lead to long-term behavioral deficits, such as mental dysfunction, particularly that related to the fear learning (predicting aversive events) associated with post-traumatic stress disorder (PTSD), of which the underlying pathology is not well understood. Thus, the proposed studies aim to use highly-translational neuroimaging techniques in mice to measure longitudinal subcellular, epigenetic (gene modifiying) regulation following mTBI within regions of the brain that regulate cognition and fear learning. Studies will also evaluate the efficacy of targeting the mTBI-induced epigenetic regulation for rehabilitation of learning and memory using a pharmacological inhibitor (drug treatment) of HDACs as a therapeutic. HDACs are a class of enzymes responsible for modifying gene expression, thereby reducing the burden of TBI-related cognitive deficits and improving quality of life for those suffering from TBI. In these studies, mice will endure certain levels of distress, but this is necessary in order to translate the findings of our studies to benefit humans in the long run.
COMORBIDITY OF PTSD AND ALCOHOL DEPENDENCE: ENDOCANNABINOID REGULATION
Combat-exposed Veterans and military personnel have a higher risk of developing posttraumatic stress disorder (PTSD) than civilians and often use alcohol to alleviate their PTSD symptoms, which can render them more susceptible to alcohol use disorders (AUDs) and subdequent withdrawal-induced disorders. It is well documented that individuals with PTSD comorbid with AUD have more intense alcohol cravings and faster relapse during withdrawal than those with AUD only, making Veterans uniquely susceptible. Although there are therapeutic treatments for either disorder alone, there is currently no treatment for the combination of PTSD and AUD. Also, the role of the cannabinoid system, a regulatory system that regulates stress and alcohol, on the comorbid PTSD-AUD is yet unknown. Through use of a mouse model, this project aims to address the long-term effects of PTSD-AUD on anxiety and ethanol consumption behaviors resulting from dyregulation of the cannabinoid system and to investigate long-lasting rehabilitation strategies, such as pharmacotherapy, which can be used upon return from active duty. Thus, these studies are highly relevant to the mission for VA patient care and rehabilitation.
TBI-INDUCED SYNAPTIC PLASTICITY: EFFECTS ON ETHANOL SENSITIVITY
Clinical studies have identified TBI as a significant risk factor for alcohol use disorders. TBI patients who relapsed to alcohol abuse had a greater frequency of brain lesions involving neural circuits that mediate aspects of addictive behavior. Preclinical studies of post-TBI dendritic spine remodeling and synaptic modification have indicated that TBI is capable of inhibiting anatomical manifestations of neuronal plasticity. Importantly, evidence of disrupted dendritic structure has also been demonstrated in rats with altered ethanol sensitivity. Likewise, rats lacking the ability to regulate synaptic organization have reduced ethanol tolerance. Increased functional recovery from TBI in rodents has been demonstrated following environmental enrichment (EE) exposure, with associated enhancement of dendritic density. EE has been shown to provide protective effects against alcohol abuse susceptibility in rodents. The overall hypothesis of this project is that non-contusive TBI will impair synaptic function leading to increased sensitivity to ethanol, which can be reversed by environmental enrichment. Specific aims: 1.) Determine the effect of non-contusive TBI on dendritic arborization and synapse formation and function in brain regions that mediate ethanol sensitivity, 2.) Determine the effect of non-contusive TBI on acute and chronic ethanol sensitivity, 3.) Evaluate the efficacy of environmental enrichment in reversing the effects of non-contusive TBI on ethanol sensitivity by improving/restoring synaptic development and function.
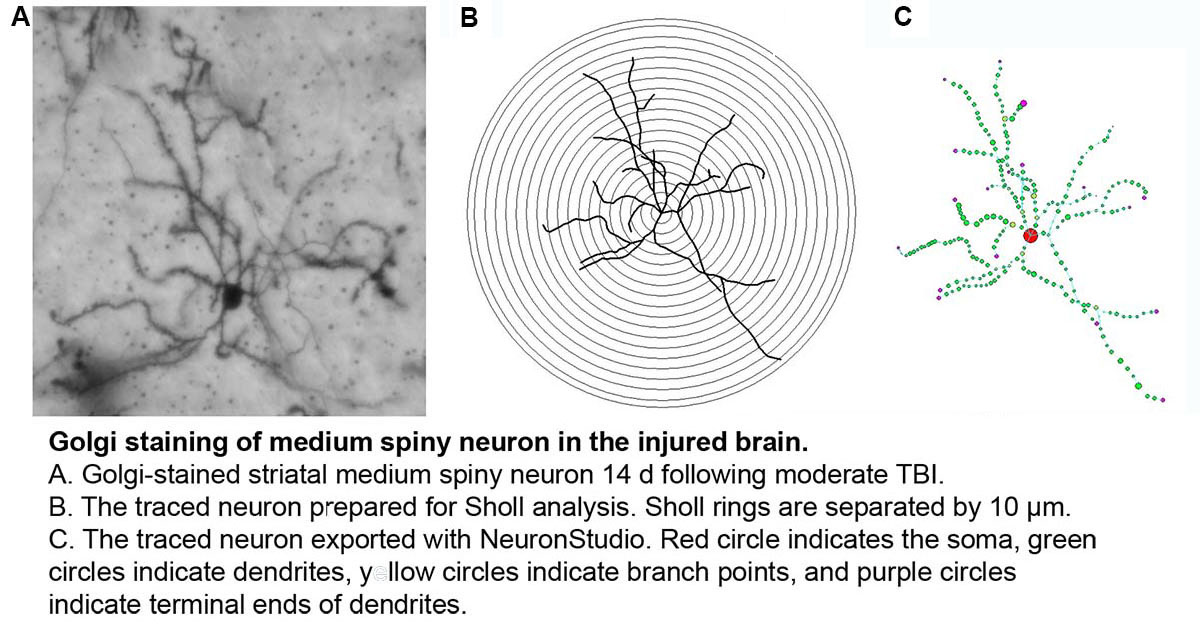
LONGITUDINAL ASSESSMENT OF CORTICAL HYPOACTIVITY IN A MODEL OF COMORBID TBI-PTSD
Recent data demonstrate that those with TBI-related injuries concurrent with PTSD, experience worsening of symptoms in the delayed post-deployment period. One mechanism to have recently come into focus to explain the compounded effects of comorbid PTSD-TBI is impaired cortical activation, a condition that has been observed following either TBI or PTSD alone. Cortical hypoactivation in comorbid PTSD-TBI, will be evaluated using mouse models of mild, non-contusive TBI and PTSD at numerous levels, including markers of excitatory neurotransmission, dendritic structure, cortical volume, neuronal activation and cortically-mediated behaviors. Specific Aims: 1.) Evaluate the influence of comorbid PTSD-TBI on cognitive behaviors, neuronal structure and markers of excitatory neurotransmission compared to either condition alone, 2.) Quantify the long-term impacts of comorbid PTSD-TBI on cortical neurotransmitter levels (neurochemical), volume (structural) and neuroactivation (functional) using novel magnetic resonance (MR) imaging techniques in a longitudinal manner in vivo compared to either condition alone, 3.) Evaluate the ability of neurotherapeutic treatment to improve/restore structural and neurotransmitter-related deficits associated with PTSD-TBI and thereby, to improve cognitive behaviors and cortical function.
ADENYLYL CYCLASES 1 AND 8 AS MEDIATORS OF DRUG- AND ETHANOL-INDUCED NEUROPLASTIC DEFICITS
This area of research interest is focused on defining the role of calcium/calmodulin-stimulated adenylyl cyclase (AC) enzymes, AC1 and 8, in the behavioral sensitivity and neurochemical responses to drugs of abuse. The first project identified deficits in behavioral sensitization and striatal dopaminergic responses to methamphetamine in mice genetically deficient in both AC1 and 8 (DKO mice). The second project extended this line of research to show impaired dopaminergic signaling, as well as acute and sensitized locomotor activity, stimulated by ethanol in DKO mice. To dissect the isoform specificity and post-synaptic target mediating the influence of calcium-stimulated ACs on ethanol action, a third project demonstrated a selective role for AC1 in ethanol-induced neuroadaptive effects on locomotor sensitization and striatal glutamate NMDA receptor function. Loss of AC1 signaling, by genetic deletion or pharmacological inhibition, was found to reduce voluntary ethanol consumption and preference in animal models, enhancing its translational relevance in rehabilitation strategies for addiction.
To study the impact of calcium-stimulated ACs on developmental ethanol exposure, another major project found enhanced vulnerability for behavioral alterations in adolescence and adulthood following acute early-life alcohol treatment, which models neurotoxicity observed in human fetal alcohol syndrome.
A final project has utilized a translationally-relevant imaging technique, manganese-enhanced MRI to detect baseline neuronal activity changes across several reward-related brain regions in DKO mice in correlation with altered reward-associated drinking behavior.
EFFECTS OF TBI ON ALCOHOL-RELATED BEHAVIORS- ROLE OF THE CANNABINOID SYSTEM
This project is designed to evaluate the effects of TBI on alcohol consumption and withdrawal behaviors and the mechanisms that subserve them, in particular the role of the cannabinoid (CB) system. TBI is known to dysregulate CB signaling, a pathway germane to alcohol action in the brain. Chronic, binge, sensitization and alcohol preference models will be evaluated after TBI, as well as endocannabinoid and CB receptor expression in the anterior striatum.
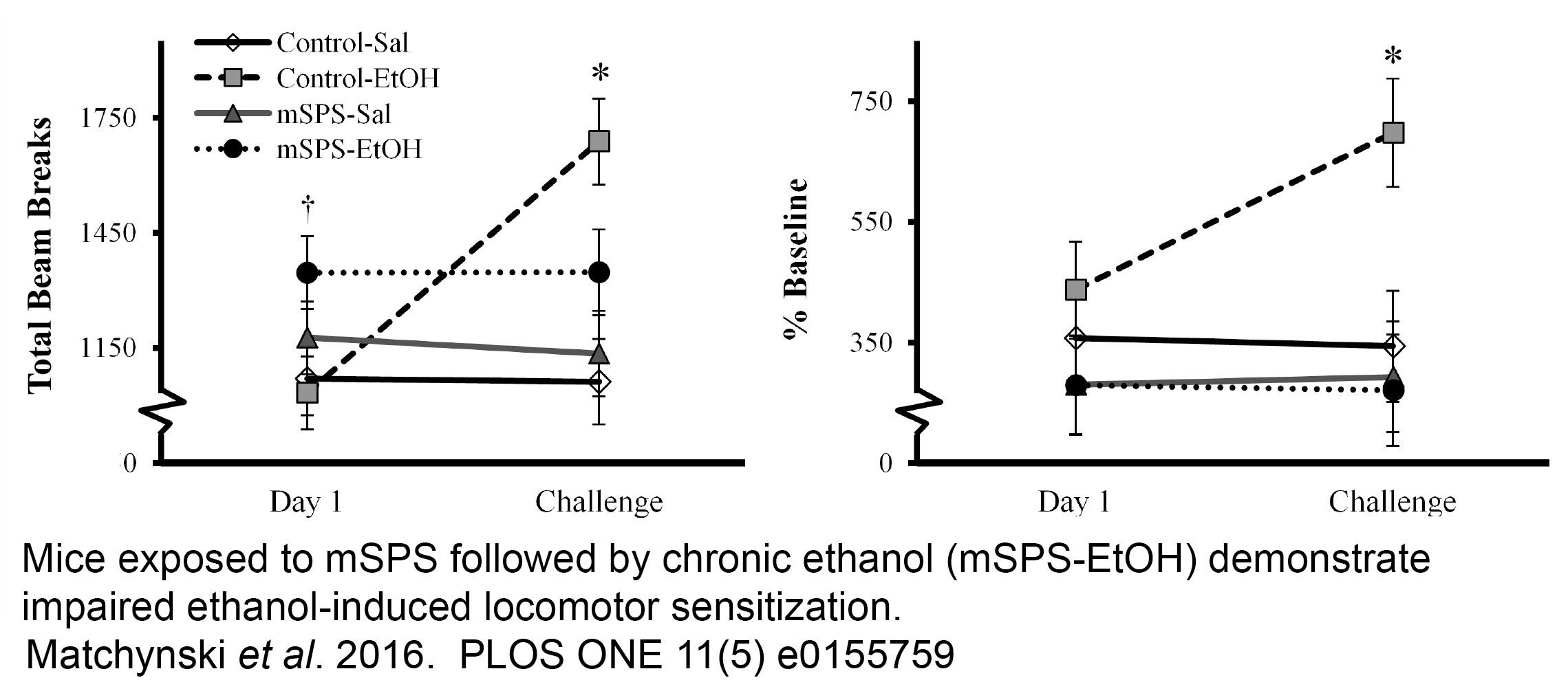
EFFECTS OF TRAUMATIC STRESS AS A MODEL OF PTSD ON ALCOHOL-RELATED BEHAVIORS- ROLE OF THE CANNABINOID SYSTEM
Using a model of PTSD in the mouse, single prolonged stress (mSPS), this project is designed to evaluate the effects of traumatic stress on alcohol consumption and withdrawal behaviors and the mechanisms that subserve them, in particular the role of the cannabinoid (CB) system. Chronic, binge, sensitization and alcohol preference models have been used to examine cannabinoid receptor 1 expression in the anterior striatum revealing a synergistic effect of stress and chronic alcohol exposure to reduce CB1 expression that is correlated with impaired striatal neuroplasticity.
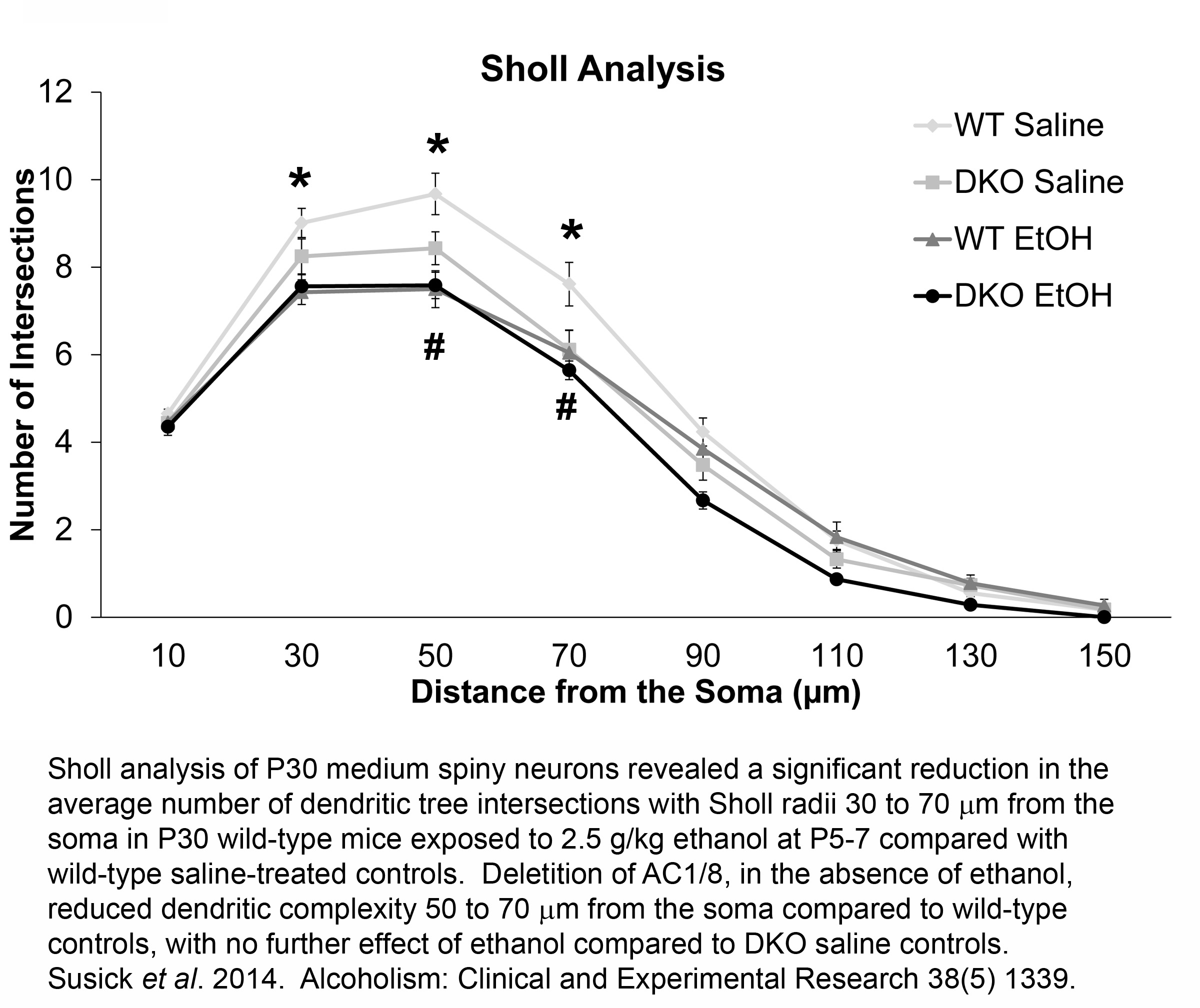
FETAL ALCOHOL DISORDERS AND ADENYLYL CYCLASES 1/8
Neonatal alcohol exposure in rodents causes dramatic neurodegenerative effects throughout the developing nervous system, particularly in the striatum, acutely after exposure. These acute neurodegenerative effects are augmented in mice lacking adenylyl cyclases 1 and 8 (AC1/8) as neonatal mice with a genetic deletion of both AC isoforms (DKO) have increased vulnerability to ethanol-induced striatal neurotoxicity compared to wild type (WT) controls. To further our understanding of the negative effects of ethanol, we analyzed neuron morphology and spine density of medium spiny neurons at P30 from WT and DKO mice treated with 2.5g/kg ethanol at P5-7, two synaptic proteins, PSD-95 and synaptophysin, BDNF-related signaling proteins including BDNF, pro-BDNF, the BDNF receptor, TrkB, Akt, ERK, and PLCÉ£1, and striatal-dependent behaviors at various time points (2, 4, 6, 24, 48 h, P14, P21, and P30) following neonatal ethanol exposure.